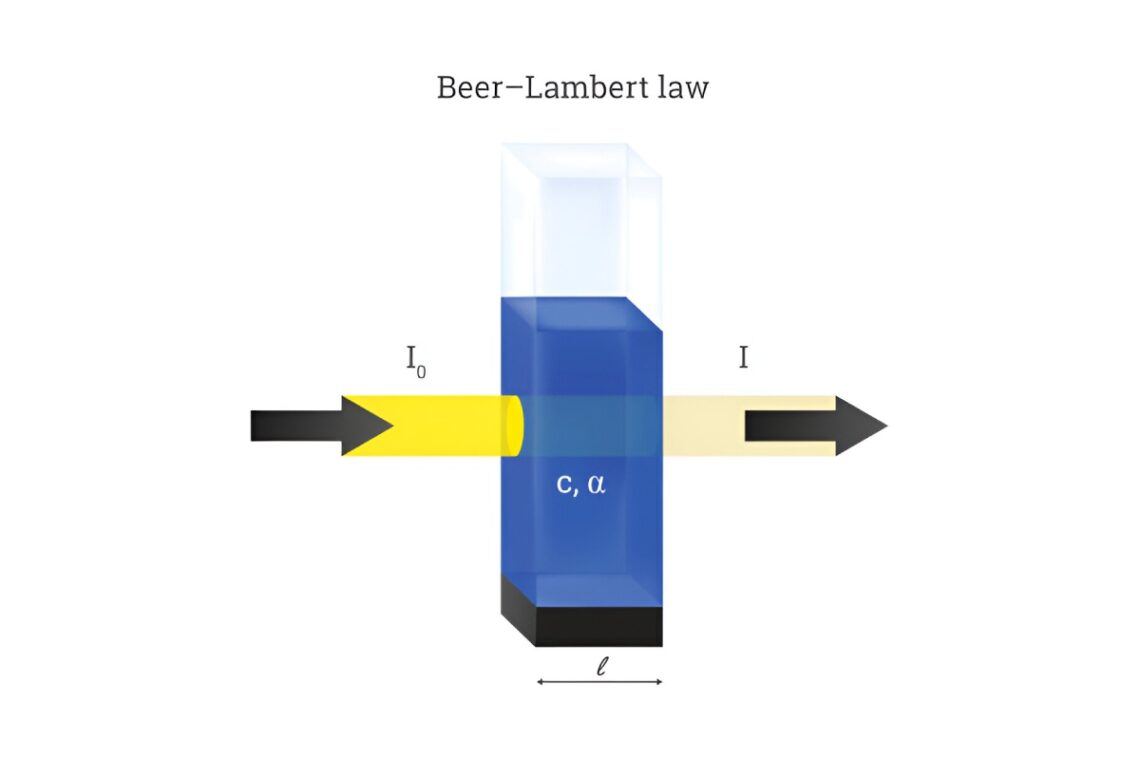
The Beer Lambert Law is a fundamental principle in the field of spectroscopy that explains the relationship between light absorption and the Concentration of a substance. This law is crucial in chemistry, biology, physics, and environmental science. Whether you’re a student, researcher, or just curious about how scientists measure substances using light, understanding the Beer Lambert Law is essential.
In this article, we’ll explain the Beer Lambert Law, how it works, its formula, and its applications in real-world science.
What is Beer Lambert Law?
The Beer Lambert Law, sometimes called Beer’s Law, describes how the Absorbance of light by a solution is directly proportional to the Concentration of the absorbing substance and the path length through which the light travels.
In simpler terms:
The more concentrated a solution is, the more light it absorbs.
This principle allows scientists to calculate the Concentration of a solute in a solution just by measuring how much light passes through it.
History of the Law
The law is named after:
- August Beer, who formulated the proportional relationship in 1852
- Johann Heinrich Lambert, who described how light intensity decreases as it travels through an absorbing medium in the 18th century
Together, their work led to what we now call the Beer-Lambert Law.
Beer Lambert Law Formula
The standard equation for the Beer-Lambert Law is:
A = ε × c × l
Where:
- A = Absorbance (no units)
- ε = Molar absorptivity (L mol⁻¹ cm⁻¹) – a constant specific to each substance
- c = Concentration of the solution (mol/L)
- l = Path length of the light through the solution (cm)
This formula shows that Absorbance increases with both concentration and path length.
Important Notes:
- Absorbance has no units because it’s a ratio (logarithmic measurement of light intensity)
- The path length is usually 1 cm in standard cuvettes
- Molar absorptivity depends on the chemical and the wavelength used
How Does Beer-Lambert Law Work?
When a beam of light passes through a solution:
- The solute molecules absorb some of the light
- The rest pass through and remain detected by a sensor
A spectrophotometer shines light at a specific wavelength and measures how much light is absorbed. By applying the formula, we can calculate the unknown Concentration of the solution.
It benefits colored solutions or substances that absorb ultraviolet or visible light.
Real-World Applications of Beer Lambert Law
Chemical Analysis
In chemistry labs, It is used to determine concentrations of substances in:
- Reactions
- Unknown solutions
- Quality control in industries
Medical Diagnostics
It is used in medical tests, such as:
- Measuring hemoglobin levels in blood
- Glucose monitoring
- Detecting biomarkers in urine or serum
Environmental Science
The law is applied to analyze pollutants in:
- Drinking water
- Air samples
- Soil leachates
Pharmaceutical Industry
Drug concentration and purity are often checked using absorbance techniques based on it during manufacturing and quality control.
Biology and Biochemistry
It’s commonly used to:
- Quantify DNA and RNA concentration
- Measure protein absorbance at 280 nm
- Monitor enzyme activity
Limitations of Beer Lambert Law
It is extremely useful, it has limitations:
- Only works at low concentrations – At higher concentrations, molecules may interact and affect the absorption
- Applies only to monochromatic light – It’s most accurate when a single wavelength remain used
- Does not account for scattering – Suspended particles or turbidity in the solution can distort the results
- Requires a uniform path length – Inconsistent cuvette dimensions can lead to errors
Tips for Accurate Results
- Always use clean, scratch-free cuvettes
- Calibrate the spectrophotometer before measurements
- Choose the wavelength at which the substance absorbs the most (λ max)
- Prepare a blank solution to set baseline absorbance to zero
- Use standard solutions to create a calibration curve when needed
Final Thoughts
The Beer-Lambert Law is a simple yet powerful tool in modern science. It bridges the gap between light and matter, allowing precise, non-invasive measurement of substance concentrations. This law plays a foundational role in scientific analysis, from medical labs to environmental testing.
By understanding how Absorbance relates to concentration and path length, researchers and students can make accurate predictions, analyze samples, and monitor changes over time.
Whether you’re working in a lab or studying science in school, mastering and it’s can give you a better understanding of how light helps us uncover the invisible world of chemistry.